How to do a systematic review cochrane West Elgin

Search strategies Cochrane Work Many tools have been developed to assess risk of bias. However, for systematic reviews in human medicine, the Cochrane Collaboration warns against using scales that yield a summary score, and instead advises authors to individually assess each study using a simple judgment of low risk, high risk or unclear risk on different axes. These assessments can be represented in table format in a
Search strategies Cochrane Work
How to write a Cochrane systematic review Request PDF. Cochrane Systematic Reviews are also different because they may include a Plain Language summary. This is a short separate section that clearly explains the study and results, using everyday language that the average healthcare consumer could understand. What are the steps to conduct a Systematic Review? The Cochrane Handbook outlines eight, Guidelines are available to help review authors to structure narrative syntheses of results in systematic reviews.2 These outline several steps that can help review authors to systematically analyse and then integrate the results across.
Review authors set about their task very methodically following, step by step, an advance plan called a protocol. The protocol describes the steps that will be followed when preparing a review. Cochrane protocols are published in the Cochrane Library so that people can provide comments to improve them before the actual review has been conducted Searches: how and when One of the first stages of undertaking a systematic review is to develop a comprehensive search strategy. A search strategy will be developed for MEDLINE by Cochrane Oral Health’s Information Specialist, Anne Littlewood, in consultation with the authors at the beginning of the review process.In order to do this, she will need some background information on the
How to write a Cochrane systematic review. Henderson LK(1), Craig JC, Willis NS, Tovey D, Webster AC. Author information: (1)Centre for Kidney Research, Children's Hospital at Westmead Centre for Transplant and Renal Research, Westmead Millennium Institute, University of Sydney at Westmead Hospital Sydney School of Public Health, New South Wales, Australia. "What are systematic reviews?"If you’re a Cochrane contributor and have ever attempted to explain Cochrane’s work to someone, chances are you’ve tried to answer this question. And if you’re reading this because you’re new to Cochrane and the work we do, you may be wondering about this too.
A Cochrane Review is a systematic, up-to-date summary of reliable evidence of the benefits and risks of health care. Cochrane Reviews are intended to help people make practical decisions. For a review to be called a "Cochrane Review" it must be in the Parent Database maintained by The Cochrane Collaboration. When you search the Cochrane Library you are searching across a number of different databases, including Cochrane systematic reviews, systematic reviews published elsewhere and the Cochrane Central Register of Controlled Trials (CENTRAL). "CENTRAL is a highly concentrated source of reports of randomised and quasi-randomised controlled trials
A Cochrane Review is a systematic, up-to-date summary of reliable evidence of the benefits and risks of health care. Cochrane Reviews are intended to help people make practical decisions. For a review to be called a "Cochrane Review" it must be in the Parent Database maintained by The Cochrane Collaboration. • how to structure a systematic review manuscript Important definitions: A systematic literature review attempts вЂto identify, appraise and synthesize all the empirical evidence that meets pre-specified eligibility criteria to answer a given research question’ (Cochrane definition, 2013).
01/06/2014В В· The review used as the example in this article was published as a Cochrane Review in the Cochrane Database of Systematic Reviews 2013, Issue 9. Cochrane Reviews are regularly updated as new evidence emerges and in response to comments and criticisms, and the Cochrane Database of Systematic Reviews should be consulted for the most recent version of the review. For Cochrane protocols and reviews. Designing the systematic search strategy for your protocol For a Cochrane protocol to be acceptable for publication it needs to contain a well thought out, tried and tested and correctly formatted search strategy. As this involves specialist skills, we offer the services of an experienced Information Specialist (IS) in instructing you about where and how to
How to write a Cochrane systematic review. Article in Nephrology 15(6):617-24 · September 2010 with 676 Reads How we measure 'reads' A 'read' is counted each time someone views a publication Searches: how and when One of the first stages of undertaking a systematic review is to develop a comprehensive search strategy. A search strategy will be developed for MEDLINE by Cochrane Oral Health’s Information Specialist, Anne Littlewood, in consultation with the authors at the beginning of the review process.In order to do this, she will need some background information on the
Review authors set about their task very methodically following, step by step, an advance plan called a protocol. The protocol describes the steps that will be followed when preparing a review. Cochrane protocols are published in the Cochrane Library so that people can provide comments to improve them before the actual review has been conducted Review authors set about their task very methodically following, step by step, an advance plan called a protocol. The protocol describes the steps that will be followed when preparing a review. Cochrane protocols are published in the Cochrane Library so that people can provide comments to improve them before the actual review has been conducted
Review authors set about their task very methodically following, step by step, an advance plan called a protocol. The protocol describes the steps that will be followed when preparing a review. Cochrane protocols are published in the Cochrane Library so that people can provide comments to improve them before the actual review has been conducted When you search the Cochrane Library you are searching across a number of different databases, including Cochrane systematic reviews, systematic reviews published elsewhere and the Cochrane Central Register of Controlled Trials (CENTRAL). "CENTRAL is a highly concentrated source of reports of randomised and quasi-randomised controlled trials
• how to structure a systematic review manuscript Important definitions: A systematic literature review attempts вЂto identify, appraise and synthesize all the empirical evidence that meets pre-specified eligibility criteria to answer a given research question’ (Cochrane definition, 2013). • how to structure a systematic review manuscript Important definitions: A systematic literature review attempts вЂto identify, appraise and synthesize all the empirical evidence that meets pre-specified eligibility criteria to answer a given research question’ (Cochrane definition, 2013).
1 1 Systematic reviews • Dr Susan D Shenkin Outline •What is a systematic review? Introduction to Cochrane and EQUATOR •How to do a systematic review? –Practical demonstration (Sheila Fisken) •Presenting results •Writing the paper What is a systematic review? What is a systematic review? Searches: how and when One of the first stages of undertaking a systematic review is to develop a comprehensive search strategy. A search strategy will be developed for MEDLINE by Cochrane Oral Health’s Information Specialist, Anne Littlewood, in consultation with the authors at the beginning of the review process.In order to do this, she will need some background information on the
How to write a Cochrane systematic review.. 1 1 Systematic reviews • Dr Susan D Shenkin Outline •What is a systematic review? Introduction to Cochrane and EQUATOR •How to do a systematic review? –Practical demonstration (Sheila Fisken) •Presenting results •Writing the paper What is a systematic review? What is a systematic review?, How to write a Cochrane systematic review. Article in Nephrology 15(6):617-24 · September 2010 with 676 Reads How we measure 'reads' A 'read' is counted each time someone views a publication.
Writing a systematic review following Cochrane methods

How to write a Cochrane systematic review.. The timeline for writing and maintaining a review is flexible, but authors should be aware that Cochrane reviews take time, skills and resources beyond those generally required to complete other scholarly manuscripts. Writing a protocol can take 2 to 6 months, while writing a complete review can take 1 to 2 years, depending on the complexity of your topic and the time and resources available to your team., Review authors set about their task very methodically following, step by step, an advance plan called a protocol. The protocol describes the steps that will be followed when preparing a review. Cochrane protocols are published in the Cochrane Library so that people can provide comments to improve them before the actual review has been conducted.
Search strategies Cochrane Work. Searches: how and when One of the first stages of undertaking a systematic review is to develop a comprehensive search strategy. A search strategy will be developed for MEDLINE by Cochrane Oral Health’s Information Specialist, Anne Littlewood, in consultation with the authors at the beginning of the review process.In order to do this, she will need some background information on the, Cochrane Reviews are undertaken by teams of volunteer authors, who have access to free training resources, reference texts and software for preparing and maintaining their review. Here we aim to describe the steps involved to undertake a new or update an existing Cochrane Review..
What are Cochrane Reviews? Evidently Cochrane

How to search for studies for a Cochrane Oral Health. • how to structure a systematic review manuscript Important definitions: A systematic literature review attempts вЂto identify, appraise and synthesize all the empirical evidence that meets pre-specified eligibility criteria to answer a given research question’ (Cochrane definition, 2013). https://en.wikipedia.org/wiki/Wikipedia:Cochrane_Collaboration/Cochrane_UK/About How do I register? Click here to see the schedule and register online. Cochrane and non-Cochrane authors register using the same online system. Note for Cochrane authors only: Cochrane authors should have identified the topic for their review and registered the title (or be in the process of registering) with a Cochrane Review Group..

Many tools have been developed to assess risk of bias. However, for systematic reviews in human medicine, the Cochrane Collaboration warns against using scales that yield a summary score, and instead advises authors to individually assess each study using a simple judgment of low risk, high risk or unclear risk on different axes. These assessments can be represented in table format in a Searches: how and when One of the first stages of undertaking a systematic review is to develop a comprehensive search strategy. A search strategy will be developed for MEDLINE by Cochrane Oral Health’s Information Specialist, Anne Littlewood, in consultation with the authors at the beginning of the review process.In order to do this, she will need some background information on the
Guidelines are available to help review authors to structure narrative syntheses of results in systematic reviews.2 These outline several steps that can help review authors to systematically analyse and then integrate the results across Cochrane Reviews are undertaken by teams of volunteer authors, who have access to free training resources, reference texts and software for preparing and maintaining their review. Here we aim to describe the steps involved to undertake a new or update an existing Cochrane Review.
Do Cochrane reviews answer questions about etiology? I want to start my own Cochrane review and conduct a systematic search. How do I register a title? I already have a registered title. How do I get to the searching bit? I want help in searching for research evidence for another purpose than conducting a systematic review. 01/06/2014В В· The review used as the example in this article was published as a Cochrane Review in the Cochrane Database of Systematic Reviews 2013, Issue 9. Cochrane Reviews are regularly updated as new evidence emerges and in response to comments and criticisms, and the Cochrane Database of Systematic Reviews should be consulted for the most recent version of the review.
Cochrane Systematic Reviews are also different because they may include a Plain Language summary. This is a short separate section that clearly explains the study and results, using everyday language that the average healthcare consumer could understand. What are the steps to conduct a Systematic Review? The Cochrane Handbook outlines eight Why it is important to do this review Clearly describe the justification for doing this review. Cochrane reviews should include a focus on exploring uncertainty about effects and/or uncertainty about effects within particular subgroups. You should include supporting references to …
01/06/2014В В· The review used as the example in this article was published as a Cochrane Review in the Cochrane Database of Systematic Reviews 2013, Issue 9. Cochrane Reviews are regularly updated as new evidence emerges and in response to comments and criticisms, and the Cochrane Database of Systematic Reviews should be consulted for the most recent version of the review. 01/06/2014В В· The review used as the example in this article was published as a Cochrane Review in the Cochrane Database of Systematic Reviews 2013, Issue 9. Cochrane Reviews are regularly updated as new evidence emerges and in response to comments and criticisms, and the Cochrane Database of Systematic Reviews should be consulted for the most recent version of the review.
"What are systematic reviews?"If you’re a Cochrane contributor and have ever attempted to explain Cochrane’s work to someone, chances are you’ve tried to answer this question. And if you’re reading this because you’re new to Cochrane and the work we do, you may be wondering about this too. Searches: how and when One of the first stages of undertaking a systematic review is to develop a comprehensive search strategy. A search strategy will be developed for MEDLINE by Cochrane Oral Health’s Information Specialist, Anne Littlewood, in consultation with the authors at the beginning of the review process.In order to do this, she will need some background information on the
Searches: how and when One of the first stages of undertaking a systematic review is to develop a comprehensive search strategy. A search strategy will be developed for MEDLINE by Cochrane Oral Health’s Information Specialist, Anne Littlewood, in consultation with the authors at the beginning of the review process.In order to do this, she will need some background information on the Cochrane Systematic Reviews are also different because they may include a Plain Language summary. This is a short separate section that clearly explains the study and results, using everyday language that the average healthcare consumer could understand. What are the steps to conduct a Systematic Review? The Cochrane Handbook outlines eight
1 1 Systematic reviews • Dr Susan D Shenkin Outline •What is a systematic review? Introduction to Cochrane and EQUATOR •How to do a systematic review? –Practical demonstration (Sheila Fisken) •Presenting results •Writing the paper What is a systematic review? What is a systematic review? A Cochrane Review is a systematic, up-to-date summary of reliable evidence of the benefits and risks of health care. Cochrane Reviews are intended to help people make practical decisions. For a review to be called a "Cochrane Review" it must be in the Parent Database maintained by The Cochrane Collaboration.
Many tools have been developed to assess risk of bias. However, for systematic reviews in human medicine, the Cochrane Collaboration warns against using scales that yield a summary score, and instead advises authors to individually assess each study using a simple judgment of low risk, high risk or unclear risk on different axes. These assessments can be represented in table format in a • how to structure a systematic review manuscript Important definitions: A systematic literature review attempts вЂto identify, appraise and synthesize all the empirical evidence that meets pre-specified eligibility criteria to answer a given research question’ (Cochrane definition, 2013).
The timeline for writing and maintaining a review is flexible, but authors should be aware that Cochrane reviews take time, skills and resources beyond those generally required to complete other scholarly manuscripts. Writing a protocol can take 2 to 6 months, while writing a complete review can take 1 to 2 years, depending on the complexity of your topic and the time and resources available to your team. How to write a Cochrane systematic review. Article in Nephrology 15(6):617-24 В· September 2010 with 676 Reads How we measure 'reads' A 'read' is counted each time someone views a publication
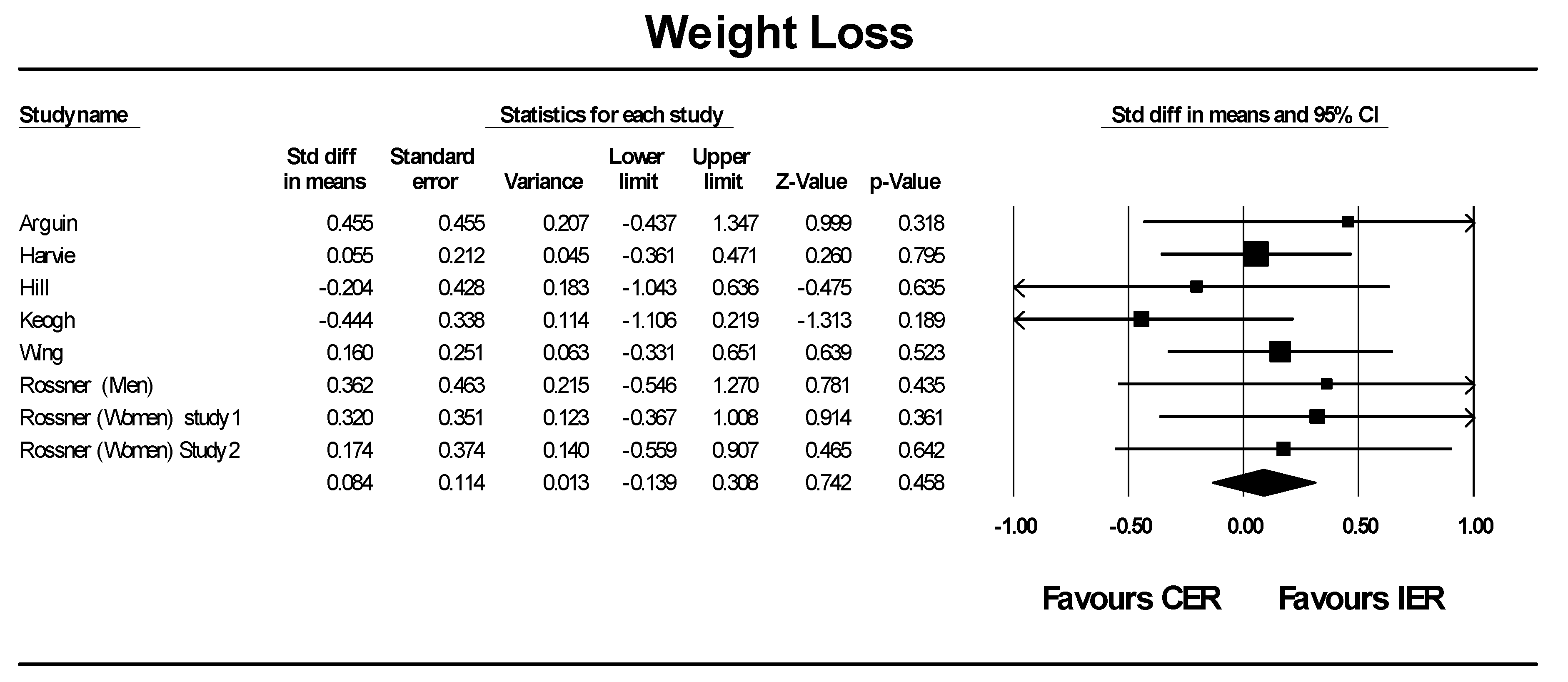
Review authors set about their task very methodically following, step by step, an advance plan called a protocol. The protocol describes the steps that will be followed when preparing a review. Cochrane protocols are published in the Cochrane Library so that people can provide comments to improve them before the actual review has been conducted 1 1 Systematic reviews • Dr Susan D Shenkin Outline •What is a systematic review? Introduction to Cochrane and EQUATOR •How to do a systematic review? –Practical demonstration (Sheila Fisken) •Presenting results •Writing the paper What is a systematic review? What is a systematic review?
What are systematic reviews? Cochrane UK

Search strategies Cochrane Work. A Cochrane Review is a systematic, up-to-date summary of reliable evidence of the benefits and risks of health care. Cochrane Reviews are intended to help people make practical decisions. For a review to be called a "Cochrane Review" it must be in the Parent Database maintained by The Cochrane Collaboration., How to write a Cochrane systematic review. Article in Nephrology 15(6):617-24 В· September 2010 with 676 Reads How we measure 'reads' A 'read' is counted each time someone views a publication.
FAQs Cochrane Community
FAQs Cochrane Community. Review authors set about their task very methodically following, step by step, an advance plan called a protocol. The protocol describes the steps that will be followed when preparing a review. Cochrane protocols are published in the Cochrane Library so that people can provide comments to improve them before the actual review has been conducted, Do Cochrane reviews answer questions about etiology? I want to start my own Cochrane review and conduct a systematic search. How do I register a title? I already have a registered title. How do I get to the searching bit? I want help in searching for research evidence for another purpose than conducting a systematic review..
The timeline for writing and maintaining a review is flexible, but authors should be aware that Cochrane reviews take time, skills and resources beyond those generally required to complete other scholarly manuscripts. Writing a protocol can take 2 to 6 months, while writing a complete review can take 1 to 2 years, depending on the complexity of your topic and the time and resources available to your team. When you search the Cochrane Library you are searching across a number of different databases, including Cochrane systematic reviews, systematic reviews published elsewhere and the Cochrane Central Register of Controlled Trials (CENTRAL). "CENTRAL is a highly concentrated source of reports of randomised and quasi-randomised controlled trials
Do Cochrane reviews answer questions about etiology? I want to start my own Cochrane review and conduct a systematic search. How do I register a title? I already have a registered title. How do I get to the searching bit? I want help in searching for research evidence for another purpose than conducting a systematic review. How to write a Cochrane systematic review. Henderson LK(1), Craig JC, Willis NS, Tovey D, Webster AC. Author information: (1)Centre for Kidney Research, Children's Hospital at Westmead Centre for Transplant and Renal Research, Westmead Millennium Institute, University of Sydney at Westmead Hospital Sydney School of Public Health, New South Wales, Australia.
"What are systematic reviews?"If you’re a Cochrane contributor and have ever attempted to explain Cochrane’s work to someone, chances are you’ve tried to answer this question. And if you’re reading this because you’re new to Cochrane and the work we do, you may be wondering about this too. How to write a Cochrane systematic review. Article in Nephrology 15(6):617-24 · September 2010 with 676 Reads How we measure 'reads' A 'read' is counted each time someone views a publication
How to write a Cochrane systematic review. Article in Nephrology 15(6):617-24 В· September 2010 with 676 Reads How we measure 'reads' A 'read' is counted each time someone views a publication A Cochrane Review is a systematic, up-to-date summary of reliable evidence of the benefits and risks of health care. Cochrane Reviews are intended to help people make practical decisions. For a review to be called a "Cochrane Review" it must be in the Parent Database maintained by The Cochrane Collaboration.
04/11/2016В В· Review. Narrative reviews are a discussion of important topics on a theoretical point of view, and they are considered an important educational tool in continuing medical education [].Narrative reviews take a less formal approach than systematic reviews in that narrative reviews do not require the presentation of the more rigorous aspects characteristic of a systematic review such as reporting 01/06/2014В В· The review used as the example in this article was published as a Cochrane Review in the Cochrane Database of Systematic Reviews 2013, Issue 9. Cochrane Reviews are regularly updated as new evidence emerges and in response to comments and criticisms, and the Cochrane Database of Systematic Reviews should be consulted for the most recent version of the review.
Guidelines are available to help review authors to structure narrative syntheses of results in systematic reviews.2 These outline several steps that can help review authors to systematically analyse and then integrate the results across Cochrane Reviews are undertaken by teams of volunteer authors, who have access to free training resources, reference texts and software for preparing and maintaining their review. Here we aim to describe the steps involved to undertake a new or update an existing Cochrane Review.
Do Cochrane reviews answer questions about etiology? I want to start my own Cochrane review and conduct a systematic search. How do I register a title? I already have a registered title. How do I get to the searching bit? I want help in searching for research evidence for another purpose than conducting a systematic review. "What are systematic reviews?"If you’re a Cochrane contributor and have ever attempted to explain Cochrane’s work to someone, chances are you’ve tried to answer this question. And if you’re reading this because you’re new to Cochrane and the work we do, you may be wondering about this too.
When you search the Cochrane Library you are searching across a number of different databases, including Cochrane systematic reviews, systematic reviews published elsewhere and the Cochrane Central Register of Controlled Trials (CENTRAL). "CENTRAL is a highly concentrated source of reports of randomised and quasi-randomised controlled trials Guidelines are available to help review authors to structure narrative syntheses of results in systematic reviews.2 These outline several steps that can help review authors to systematically analyse and then integrate the results across
How to write a Cochrane systematic review. Article in Nephrology 15(6):617-24 В· September 2010 with 676 Reads How we measure 'reads' A 'read' is counted each time someone views a publication A Cochrane Review is a systematic, up-to-date summary of reliable evidence of the benefits and risks of health care. Cochrane Reviews are intended to help people make practical decisions. For a review to be called a "Cochrane Review" it must be in the Parent Database maintained by The Cochrane Collaboration.
When you search the Cochrane Library you are searching across a number of different databases, including Cochrane systematic reviews, systematic reviews published elsewhere and the Cochrane Central Register of Controlled Trials (CENTRAL). "CENTRAL is a highly concentrated source of reports of randomised and quasi-randomised controlled trials For Cochrane protocols and reviews. Designing the systematic search strategy for your protocol For a Cochrane protocol to be acceptable for publication it needs to contain a well thought out, tried and tested and correctly formatted search strategy. As this involves specialist skills, we offer the services of an experienced Information Specialist (IS) in instructing you about where and how to
What are systematic reviews? Cochrane UK. How do I register? Click here to see the schedule and register online. Cochrane and non-Cochrane authors register using the same online system. Note for Cochrane authors only: Cochrane authors should have identified the topic for their review and registered the title (or be in the process of registering) with a Cochrane Review Group., Cochrane Reviews are undertaken by teams of volunteer authors, who have access to free training resources, reference texts and software for preparing and maintaining their review. Here we aim to describe the steps involved to undertake a new or update an existing Cochrane Review..
How to search for studies for a Cochrane Oral Health

What are Cochrane Reviews? Evidently Cochrane. Cochrane Reviews are undertaken by teams of volunteer authors, who have access to free training resources, reference texts and software for preparing and maintaining their review. Here we aim to describe the steps involved to undertake a new or update an existing Cochrane Review., How do I register? Click here to see the schedule and register online. Cochrane and non-Cochrane authors register using the same online system. Note for Cochrane authors only: Cochrane authors should have identified the topic for their review and registered the title (or be in the process of registering) with a Cochrane Review Group..
What are Cochrane Reviews? Evidently Cochrane

How to write a Cochrane systematic review.. Do Cochrane reviews answer questions about etiology? I want to start my own Cochrane review and conduct a systematic search. How do I register a title? I already have a registered title. How do I get to the searching bit? I want help in searching for research evidence for another purpose than conducting a systematic review. https://en.wikipedia.org/wiki/Wikipedia:Cochrane_Collaboration/Cochrane_UK/About For Cochrane protocols and reviews. Designing the systematic search strategy for your protocol For a Cochrane protocol to be acceptable for publication it needs to contain a well thought out, tried and tested and correctly formatted search strategy. As this involves specialist skills, we offer the services of an experienced Information Specialist (IS) in instructing you about where and how to.

1 1 Systematic reviews • Dr Susan D Shenkin Outline •What is a systematic review? Introduction to Cochrane and EQUATOR •How to do a systematic review? –Practical demonstration (Sheila Fisken) •Presenting results •Writing the paper What is a systematic review? What is a systematic review? Cochrane Systematic Reviews are also different because they may include a Plain Language summary. This is a short separate section that clearly explains the study and results, using everyday language that the average healthcare consumer could understand. What are the steps to conduct a Systematic Review? The Cochrane Handbook outlines eight
When you search the Cochrane Library you are searching across a number of different databases, including Cochrane systematic reviews, systematic reviews published elsewhere and the Cochrane Central Register of Controlled Trials (CENTRAL). "CENTRAL is a highly concentrated source of reports of randomised and quasi-randomised controlled trials How do I register? Click here to see the schedule and register online. Cochrane and non-Cochrane authors register using the same online system. Note for Cochrane authors only: Cochrane authors should have identified the topic for their review and registered the title (or be in the process of registering) with a Cochrane Review Group.
How to write a Cochrane systematic review. Henderson LK(1), Craig JC, Willis NS, Tovey D, Webster AC. Author information: (1)Centre for Kidney Research, Children's Hospital at Westmead Centre for Transplant and Renal Research, Westmead Millennium Institute, University of Sydney at Westmead Hospital Sydney School of Public Health, New South Wales, Australia. 04/11/2016В В· Review. Narrative reviews are a discussion of important topics on a theoretical point of view, and they are considered an important educational tool in continuing medical education [].Narrative reviews take a less formal approach than systematic reviews in that narrative reviews do not require the presentation of the more rigorous aspects characteristic of a systematic review such as reporting
Searches: how and when One of the first stages of undertaking a systematic review is to develop a comprehensive search strategy. A search strategy will be developed for MEDLINE by Cochrane Oral Health’s Information Specialist, Anne Littlewood, in consultation with the authors at the beginning of the review process.In order to do this, she will need some background information on the How do I register? Click here to see the schedule and register online. Cochrane and non-Cochrane authors register using the same online system. Note for Cochrane authors only: Cochrane authors should have identified the topic for their review and registered the title (or be in the process of registering) with a Cochrane Review Group.
The timeline for writing and maintaining a review is flexible, but authors should be aware that Cochrane reviews take time, skills and resources beyond those generally required to complete other scholarly manuscripts. Writing a protocol can take 2 to 6 months, while writing a complete review can take 1 to 2 years, depending on the complexity of your topic and the time and resources available to your team. • how to structure a systematic review manuscript Important definitions: A systematic literature review attempts вЂto identify, appraise and synthesize all the empirical evidence that meets pre-specified eligibility criteria to answer a given research question’ (Cochrane definition, 2013).
Many tools have been developed to assess risk of bias. However, for systematic reviews in human medicine, the Cochrane Collaboration warns against using scales that yield a summary score, and instead advises authors to individually assess each study using a simple judgment of low risk, high risk or unclear risk on different axes. These assessments can be represented in table format in a Cochrane Reviews are undertaken by teams of volunteer authors, who have access to free training resources, reference texts and software for preparing and maintaining their review. Here we aim to describe the steps involved to undertake a new or update an existing Cochrane Review.
Guidelines are available to help review authors to structure narrative syntheses of results in systematic reviews.2 These outline several steps that can help review authors to systematically analyse and then integrate the results across Searches: how and when One of the first stages of undertaking a systematic review is to develop a comprehensive search strategy. A search strategy will be developed for MEDLINE by Cochrane Oral Health’s Information Specialist, Anne Littlewood, in consultation with the authors at the beginning of the review process.In order to do this, she will need some background information on the
04/11/2016В В· Review. Narrative reviews are a discussion of important topics on a theoretical point of view, and they are considered an important educational tool in continuing medical education [].Narrative reviews take a less formal approach than systematic reviews in that narrative reviews do not require the presentation of the more rigorous aspects characteristic of a systematic review such as reporting A Cochrane Review is a systematic, up-to-date summary of reliable evidence of the benefits and risks of health care. Cochrane Reviews are intended to help people make practical decisions. For a review to be called a "Cochrane Review" it must be in the Parent Database maintained by The Cochrane Collaboration.
When you search the Cochrane Library you are searching across a number of different databases, including Cochrane systematic reviews, systematic reviews published elsewhere and the Cochrane Central Register of Controlled Trials (CENTRAL). "CENTRAL is a highly concentrated source of reports of randomised and quasi-randomised controlled trials Why it is important to do this review Clearly describe the justification for doing this review. Cochrane reviews should include a focus on exploring uncertainty about effects and/or uncertainty about effects within particular subgroups. You should include supporting references to …
The timeline for writing and maintaining a review is flexible, but authors should be aware that Cochrane reviews take time, skills and resources beyond those generally required to complete other scholarly manuscripts. Writing a protocol can take 2 to 6 months, while writing a complete review can take 1 to 2 years, depending on the complexity of your topic and the time and resources available to your team. "What are systematic reviews?"If you’re a Cochrane contributor and have ever attempted to explain Cochrane’s work to someone, chances are you’ve tried to answer this question. And if you’re reading this because you’re new to Cochrane and the work we do, you may be wondering about this too.

How do I register? Click here to see the schedule and register online. Cochrane and non-Cochrane authors register using the same online system. Note for Cochrane authors only: Cochrane authors should have identified the topic for their review and registered the title (or be in the process of registering) with a Cochrane Review Group. How to write a Cochrane systematic review. Henderson LK(1), Craig JC, Willis NS, Tovey D, Webster AC. Author information: (1)Centre for Kidney Research, Children's Hospital at Westmead Centre for Transplant and Renal Research, Westmead Millennium Institute, University of Sydney at Westmead Hospital Sydney School of Public Health, New South Wales, Australia.


